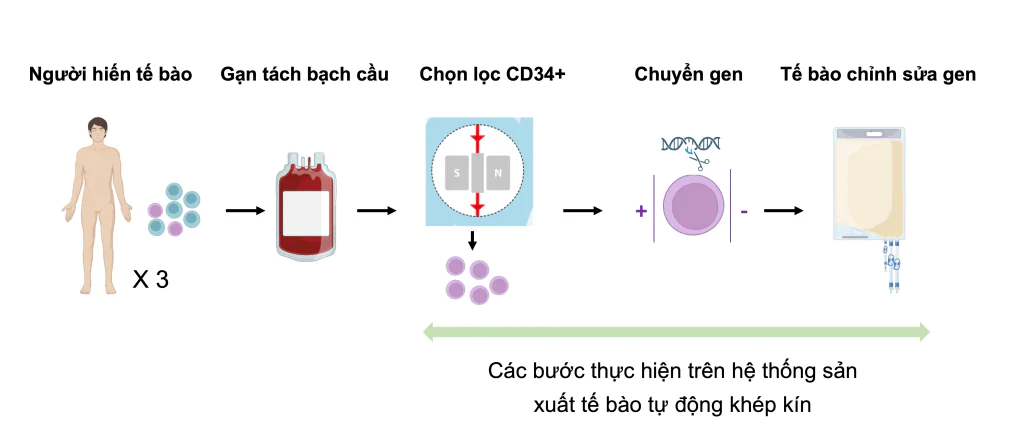
Project goals
- Deploying CRISPR-Cas9 gene editing technology on a clinical production scale on Beta Thalassemia patient cell samples.
- Evaluate the effectiveness and feasibility of the gene editing process using CRISPR-Cas9 on an automated cell production system for clinical treatment purposes. Evaluations are based on the criteria of producing a sufficient dose of cells and meeting cell quality standards for traceability.





